Have you ever undergone an endoscopy or colonoscopy procedure? These procedures have saved countless lives, enabling healthcare professionals to identify, diagnose and treat conditions in the digestive tract that would otherwise go unnoticed. Endoscopy and colonoscopy are possible thanks to decades of research and development by the medical instrumentation community to create a non-invasive way of evaluating and treating the upper and lower digestive tract.
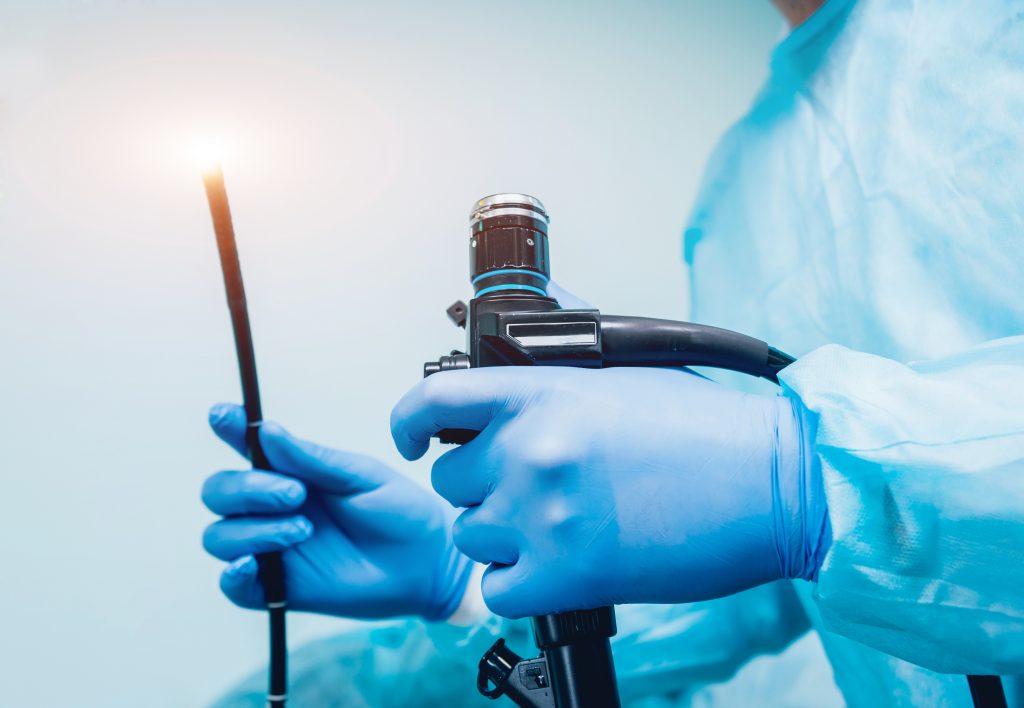 The word endoscopy is derived from the Greek words for “Looking Inside.” The first rudimentary endoscope was produced in 1806. The first significant improvement in endoscope design was the use of an electric lamp to provide illumination in 1865. The next significant improvement came with the introduction of the flexible endoscope in 1911. The first generation of the modern endoscope was developed in 1957. It used fiber optics to illuminate the subject under evaluation. Subsequent improvements included better optics for higher resolution images, brighter lighting, and the ability to remove tissue for biopsy. Today’s endoscope is a highly complex device, comprised of many parts, including lines to deliver water to the organ, and suction to evacuate the water and any samples taken for a biopsy.
The word endoscopy is derived from the Greek words for “Looking Inside.” The first rudimentary endoscope was produced in 1806. The first significant improvement in endoscope design was the use of an electric lamp to provide illumination in 1865. The next significant improvement came with the introduction of the flexible endoscope in 1911. The first generation of the modern endoscope was developed in 1957. It used fiber optics to illuminate the subject under evaluation. Subsequent improvements included better optics for higher resolution images, brighter lighting, and the ability to remove tissue for biopsy. Today’s endoscope is a highly complex device, comprised of many parts, including lines to deliver water to the organ, and suction to evacuate the water and any samples taken for a biopsy.
The success of the endoscope led to the development of the colonoscope in the early 1960s. First designed to enable examination of the lower digestive tract, it was improved to allow its use in removing polyps and tissue for biopsy. The modern colonoscope is flexible enough to allow for the complete examination of the lower intestine. Today, a colonoscopy is also prescribed regularly to screen for colon cancer.
Since endoscopes and colonoscopes are designed to be reused, there is a complex cleaning protocol to ensure their safe reuse. The Healthcare Infection Control Practices Advisory Committee (HICPAC) is a federal advisory committee chartered to provide advice and guidance to the Centers for Disease Control and Prevention (CDC) and the Secretary of the Department of Health and Human Services (HHS) regarding the practice of infection control and strategies for surveillance, prevention, and control of healthcare-associated infections, antimicrobial resistance and related events in United States healthcare settings[1]. The HICPAC developed the 6 step procedure for the effective cleaning or reprocessing of endoscopes and colonoscopes:
- Pre-cleaning
- Leak Testing
- Manual Cleaning
- Visual Inspection
- Disinfection or Sterilization
- Storage
The reprocessing can be performed manually or in automated reprocessors. Small medical facilities may choose to reprocess their scopes manually, rather than invest in an Automated Endoscope Reprocessor (AER).
There are many AERs on the market today. Some reprocess one scope at a time, and some can reprocess two scopes simultaneously. Each AER OEM uses its own protocol to affect the proper cleaning of the scopes. All must circulate the cleaning solutions and disinfectants needed to ensure the scopes are ready for another procedure. The solenoid valves typically provide the control of these solutions and disinfectants during the process cycles. To ensure that the cleaners and disinfectants flow or not at the proper time is critical to the success of the reprocessing cycle. A valve that fails to open or close at the specified time can result in cross-contamination or insufficient cleaning of the scope.
Valcor has been an integral part of the AER component market for over thirty years. One prominent AER OEM approached Valcor, looking for a reliable valve to replace the one they were using in their AER. Valcor engineers reviewed the performance of the incumbent valve, understanding the demands placed upon it, and developing a design to provide improved performance and reliability in the process. As is often typical in application development, Valcor’s engineers identified several opportunities to improve the fluid control system of the AER itself. Working closely with the OEM’s engineers, these system improvements, along with the transition to the Valcor valve vastly improved the performance and reliability of the AER. Shortly after, the OEM gave Valcor the opportunity to build the manifolds that utilize our valve. Today, Valcor provides completely assembled and tested manifolds for installation in the customer’s AER. This speeds up the assembly and test of the complete AER, providing exceptional value added to the customer.
Custom Systems and Solutions are part of our DNA. Our customers frequently let our engineers analyze their systems as part of the component application development process. We frequently recommend changes to improve the performance and reliability of the customer’s system, and often end up redesigning and manufacturing a part or all of it as a value-added turnkey package. We do the hard stuff! May we do this for you?
[1] CDC website https://www.cdc.gov/hicpac/recommendations/flexible-endoscope-reprocessing.html

Recent Comments